Novel vaccine approaches are making progress, but it will likely be years before large trials show whether they can prevent HIV.
HIV vaccine approaches that aim to train the immune system to produce specialized antibodies against the virus have taken the next steps forward, two research teams reported in Science to coincide withHIV Vaccine Awareness Dayon May 18.
Both studies showed that different engineered immunogens could trigger production of precursor immune cells with the potential to produce broadly neutralizing antibodies (bnAbs) that work against diverse strains of HIV. One study enrolled participants in Africa, which bears the brunt of the epidemic.
“We’ve now shown in humans that we can initiate the desired immune response with one shot and then drive the response further forward with a different second shot,” William Schief, PhD, of Scripps Research, said in anews release. “These trials provide proof of concept for a stepwise approach to elicit custom-tailored responses—not just for our vaccine, but for the vaccine field at large, including non-HIV vaccines.”
Scientists have spent more than three decades trying to develop vaccines to prevent HIV,with little success. Now that several traditional vaccine candidates have failed in large clinical trials, most researchers agree thatmore sophisticated approaches are needed.
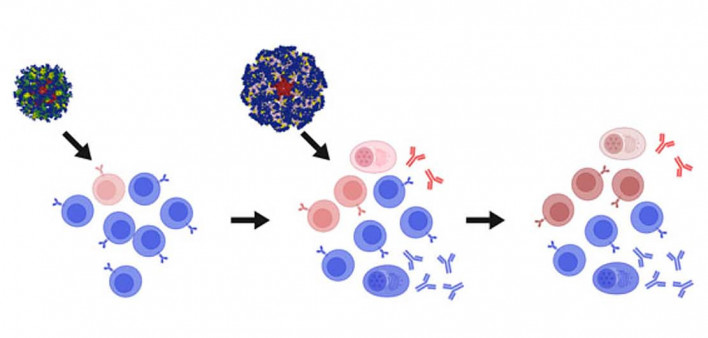
People with HIV normally produce antibodies, but these usually target parts of the virus that are highly variable, so they don’t recognize new viral mutations. Only a small proportion of individuals naturally produce bnAbs that target conserved parts of the virus, but most people do possess rare immature B cells that have the potential to do so. An approach known as germline targeting uses a series of vaccines in a stepwise manner to encourage the development of these specialized B cells and train them to produce bnAbs.
Schief’s team createdan immunogen dubbed eOD-GT8 60mer, made up of 60 engineered copies of HIV’s gp120 envelope protein fused to a nanoparticle. In 2022, the IAVI G001 trial showed that a protein-based version of the vaccinespurred production of precursor B cellsthat had the potential to produce bnAbs similar to VRC01, which targets HIV’s CD4 binding site. Last year,they reportedthat a messenger RNA (mRNA) version of a primer vaccine encoding instructions for eOD-GT8 60mer, followed by a booster dubbed core-g28v2 60mer, encouraged B-cell maturation and production of VRC01-like antibodies in mice.
This set the stage for a pair of Phase I trials: IAVI G002 (NCT05001373), which enrolled 60 participants in North America, and IAVI G003, which enrolled 18 people in Rwanda and South Africa.
As described in the first Science report, participants received the eOD-GT8 60mer mRNA primer vaccine (which Moderna calls mRNA-1644) either alone or followed by the core-g28v2 60mer booster. This so-called heterologous boosting strategy is designed to guide the immune response along the path toward bnAb production. All 17 people who received both the primer and booster developed VRC01-class antibody responses, most of whom showed “elite” responses with multiple mutations linked to bnAb development. People who received only the primer generally had less mature VRC01-class antibody responses.
“What really surprised us was the quality of the immune response we saw after just two shots—one prime and one heterologous boost,” Schief said. “We didn’t anticipate it would be that favorable.”
IAVI G003 participants received two doses of the eOD-GT8 60mer mRNA primer without the booster. All but one (94%) developed VRC01-class responses with activation of immature B cells. Interesting, they showed high levels of antibody mutation and diversity similar to what was seen in IAVI G002, despite the lack of a booster.
Inanother Phase I study, Tom Caniels, PhD, of Amsterdam University Medical Center, tested a germline-targeting approach using a trimer immunogen dubbed BG505 SOSIP.v4.1-GT1.1. Here, too, the vaccine induced production of VRC01-class bnAb precursors in a majority of recipients. What’s more, a subset of these monoclonal antibodies were structurally very similar to VRC01 and neutralized wild-type “pseudoviruses” in the laboratory.
“Across the participants we saw antibody production that tells us we’re on the right track,” senior study author Rogier Sanders, PhD, of Amsterdam University Medical Center, said in anews release. “We now know we can target the right cells with atomic precision. The next step is to further stimulate these cells to secrete broadly neutralizing antibodies.”
Yet unexpected side effects could be a concern. In the IAVI trials, the vaccines were generally well tolerated, but some participants developed mostly mild to moderate skin reactions that could be managed with antihistamines. However, about 10% of IAVI G002 participants developed chronic hives lasting six weeks or longer.
Other researchersrecently reportedthat 7% of 108 study participants who received experimental BG505 MD39.3 trimer mRNA HIV vaccines developed hives, including four with persistent symptoms lasting a year. All of those with hives had previously gotten a Moderna COVID-19 vaccine. The mechanism underlying these reactions is unclear, and further analysis is underway.
Taken together, these findings are promising, but they are still early steps in a long process. Researchers have not yet tested whether the novel vaccine approaches protect animals exposed to HIV (or its simian cousin SIV), much less whether they can prevent HIV acquisition in people.
What’s more, vaccine trials have gotten harder now that highly effective pre-exposure prophylaxis (PrEP) is widely available—withtwice-yearly lenacapaviron the way—and complex vaccine regimens that require multiple doses may not be feasible in the real world.
While an effective and globally accessible HIV prevention vaccine is a high bar, germline targeting might also play a role in therapeutic vaccines that generate bnAbs in people living with HIV, which potentially could be part of a combination strategy for long-term remission, or a functional cure.
Click here for more news aboutHIV vaccines.